Phan Thi Hiền, “Khử khuẩn dụng cụ nội soi”, Nội soi thực quản-dạ dày tá tràng trẻ em, Nhà xuất bản Y học, tháng 1 năm 2019: 34-46.
INTRODUCTION
Equipment contaminated can transmit infection from one patient to another andeven to staff. Germs may be pathogenic or opportunistic germs from other patient in the patient immunocompromised. Probe and adequate cleaning is a major concern for the safety of patient and staff. The guidelines about this problem always recommend and made modifications in recent years by several societies.
1. LEGAL RULES
Endoscopic devices disinfectant is submitted to local laws. For example, in Europe, they must be in conformity with the European rules and critical medical devices single-use.
2. CATEGORIES OF MEDICAL DEVICES
Three categories to which medical devices can be assigned are critical, semicrical, noncritical) [1].
2.1. Noncritical items
Noncritical medical devices includes:floors, walls, blood pressure cuffs, and furniture) come into contact with intact skin but not with mucous membranes [1].
2.2. Semicritical items
Semicritical medical devices come into contact with intact mucous membranesor skin that is not intact. In general, these items should be free of all microorganisms, with the exception of small numbers of bacterial spores. Flexible scopes (gastroscope, duodenoscopy, and colonoscope) are referred to as semicritical or class II. They need a high level of disinfection with mycobactericidal, viricidal, and sporicidal activity, the necessary exposuse time being determined by time it takes to inactivate 10⁶ resistant nonspore-forming test micro-organisms, including, for example, hepatitis B virus, human immunodeficiency virus (HIV), mycobacterium tuberculosis bovis [1].
2.3. Critical items
Critical medical devices have a high risk of infection because they penetrate skin and mucosa or come into contact with normally sterile tissue or the vascular system. These items need sterilization when possible, including grasping forceps, polypectomy snares, injection needles and cytology brushes should be sterilized or disposed (use singer) [1]. The law in France regulates the use single-use grasping forceps, polypectomy snares, injection needles, …
3. DISINFECTANT CHEMICALS
3.1. Glutaraldehyde 2%
Two percent glutaraldehyde is the most widely used product. At room temperature, it inactivates most bacteria in 1 minute, HIV in 2 minutes and hepatitis B virus less than 5 minutes. For hepatitis C virus, the risk persists because ribonucleic acid can be present in operating channel and on biopsy forceps, end a longer washing time (20 minutes) is necessary. The same washing time will eliminate microbacteria, in particular M.tuberculosis. Although this risk has not been described in digestive endoscopy, it is important to remember that some equipment can be shared with bronchoscopes, a device that can be in contact with such germs [1].
Glutaraldehydecreates frequent adverse reactions that can be severe as allergy, dermatitis, conjunctivitis, and rhinitis and asthma in staff and colitis in patient, owing to improperly cleaned scopes, have been made. To limit these disadvantages, procedure take place in a dedicated room, well ventilated with minimum of 12 volumes/h [1].
3.2. Peracetic acid
A suitable alternative is peracetic acid (0,2-0,35%) which has the advantage of being less of irritant; however, it is more expensive and less stable than glutaraldehyde. It acts by releasing free oxygen and hydroxyl radicals. It has rapid activity against vegetative bacteria, mycobacteria, fungi and viruses. It has bactericidal and viricidal activity after 5 minutes and skills spores and mycobacteria in 10 minutes. This product can cause cosmetic damage to the scope [1].
3.3. Others disinfectant chemicals
Chloride acid and superoxide water are highly microbicidal and are used in some units. The ASGE (American Society for Gastrointestinal Endoscopy) recommends a final rinse with 70% alcohol for its drying effect on the channels [1].
Tap water is usable for manual washing of semicritical scopes, but sterile water is necessary for the cleaning of critical ones. For the washer of disinfectors, the water has to be of level II (<10 opportunistic microorganisms/100ml at 22° and 37°C, without Pseudomonas aeruginosa). This quality of water is obtained by using filters (0,2-0,5µm) [1].
4. CONTROL PROGRAMMES FOR DISINFECTION
4.1. Tap water
Tap water should be controlled on regular basis, in particular for the presence of P. aeruginosa. Final rinse of the scope requires a microbiologic quality of level I: (100 opportunistic microorganisms/100ml at 22° and 37°C, without Pseudomonas aeruginosa).
4.1. Endoscopes
Regular cheking (one per trimester) of scope is necessary by swabs at the level of canals, exits, outer sheath, and biopsy cap. This allows the identification of poor technique and the modification of clinical practice. In case of contamination after glutaraldehyde sterilization, 50ml of sterile mixture containing Tween 80-lecithin is injected in each canal or 200ml from a common irrigator. If the bottles of liquid lavage, the cleaning water, and the knobs are autoclaved, the vehicle responsible for the contamination is often the biopsy channel. A new washing cycle needs to performed, including an alkaline enzymatic washing product for 15 minutes and a phase of sporicidal disinfection for 60 minutes. If the problems persist, the scope should be sent to the manufacturer. The remaining water in the washer or disinfectors needs to be checked monthly.
After endoscopy, there are information related to Creutzfeldt-Jakcob disease and neurodegenerative disease. Therefore, endoscopy should be reconsidered and avoided whenever possible. So, standard cleaning and high-level disinfection protocol would be adequate for reprocessing [1].
5. CLEANING OF THE SCOPES
5.1. Manual
In the past, there was a type of non-immersible endoscopes, but today it has been completely replaced by a type of immersible endoscope with video help optimizes the disinfecting process. The disinfection is carried out after each patient. It is necessary to make a list to allow alternation of endoscope and diminish waiting time and the risk of contamination[1].
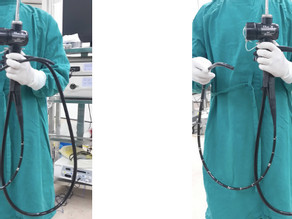
In the endoscopy room, still connected to the light source, air and water channels are flushed with water for at least 15 seconds to remove all organic material, blood to any germs with before disinfection. Prolonged exposure to these products create plaque proteins, resulting in debris that drags microorganisms to the channels or out of the endoscope. The instrument is then disconnected, a cleaning brush is passed through the suction or biopsy channels until it comes back visually clean [1].
The instrument is tested for leaks and checked for faults or damage before being fully immersed in neutral or enzymatic detergent. All removable parts (water or air, suction valves, and biopsy cap) are removed, the scope is washed, and the distal end, knobs and valves are individually scrubbed with soft toothbrush. An all channel irrigator is put in place and the channels are irrigated with the solution (minimum of 150ml) for at least 5 minutes. This step is crucial because it markedly reduces microbial contamination load and contamination of multiple-use forceps. The scope and accessories are rinsed under fresh tap water and the channels are irrigated (minimum 300ml) before air is insufflated to remove fluid residue. All of the equipment is immersed in the disinfectant for correct contact time, depending on the level of disinfection that needs to be achieved. According to the American Society of Gastrointestinal Endoscopy (ASGE) and French Society of Digestive Endoscopy (FSGE), with 2% gluataraldehyde 10-20 minutes for intermediate level and 60 minutes for high level. According to the ASGE, FSDE and Asia-Pacific Congress of Digestive Endoscopy, 10-20minutes before/after storage with 2% gluataraldehyde; with peracetic acid, 5 minutes for intermediate level and 10 minutes for high (sporicidial) level. Endoscopes and vales are rinsed with a large volume of water, 300ml passed through the channel to avoid toxic reactions (colitis). Once more, the argent is eliminated by air insufflation. Tap water, preferable with filter, is use for intermediate level and sterile water for high level of disinfection. Endoscopes are connected to the light source and forced air dried and stored hanging vertically in a designated ventilated cupboard. Valves and the biopsy cap are removed and lubricated with silicone oil [1]. Lock the angulations when the endoscopesare hanging.
Reprocessing of endoscope with video by hand includes the following steps: Precleaning Leak test Cleaning Rinsing Disinfection Rinsing Drying Storage[2].
5.1.1. Precleaning(in endoscopy room)
- Immediately after removing the endoscope from the patient, wipe the insertion tube with the clean cloth, then suck the solution cidezyme (detergent solution in small cup 500ml) and flush air-water channel within 15-30 seconds [3].
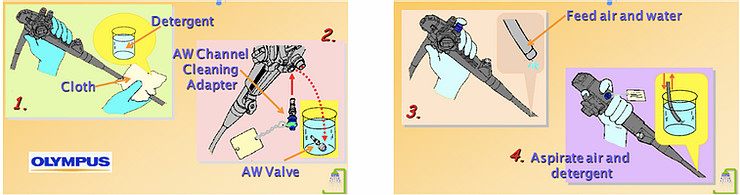
5.1.2. Leak test (after each patient) in disinfection room
- Remove valves and buttons.
- Connect the leak tester to the light source.
- Turn on the light source for maximum inflatable air.
- Connect the leak tester to the endoscope.
- Submerse the endoscope into the water and observe in 30 seconds, check the vapor at the distal bending of the endoscope.
- After the leak test: remove endoscope from the water, turn off the light source, remove the leak tester from the light source first, then check the distal bending of the endoscope until the inflation is over, finally remove the leak tester from the scope [2, 3].

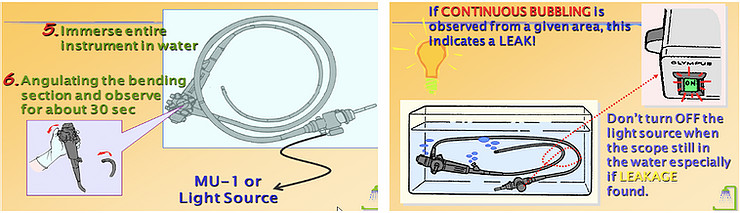
* Note: Remove the endoscope from service if a leak has been identified or detectedand follow the endoscope manufacturer’s instructions on how to procced.
5.1.3. Cleaning
When the leak test is negative. This step is the most critical step before disinfection by hand or disinfector.
- Submerged the endoscope in the basin or sink of detergent solution (dilute and used time according to the manufacturer's instructions).
- Use cloth to clean outside the endoscope cleaning.
- Use a short brush to clean all valve holes, valves, distal tip of endoscope, interventional instruments (if reused).
- Use long brush to clean interventional channel and suction channel (after each passage), rinse the brush in the detergent solution until it is clean.
- Washing tube rinse interventional channel. Use the pipe cleaning connectorand syringe 30ml inject the solution for at least 300ml into each channel, the inflate of air to push all the solution out of channels [1].
5.1.4.Rinsing
- Submerged the endoscope in the basin of clean water, use the pipe cleaning connector and syringe 30ml inject the solution for at least 300ml into each channel, then inflate to push all the water out of the endoscope.
- Rinse interventionaltools and send to sterilization at the infection control department.
5.1.5. High level of disinfection
- If this solution is used repeatedly or diluted, it will not guarantee quality. Therefore, the quality-test of disinfectant solution should be performed before immersion [2].
- Dry endoscope before immersion.
- Completely immerse the endoscope and in a high-level disinfectant solution, use the pipe cleaning connector andflush disinfectant into the biopsy channel, suction channel and air-water channel.
- Remove the the pipe cleaning connector and soakthe endoscope in the time according to the manufacturer's instructions. Remove old gloves. Use an electronic clock and record the soaking time on the board or use a timer.
- After the endoscopic has soaked in a sufficient time, use the pipe cleaning connector to inflate to push all disinfectant solution out of eachchannel, then remove the endoscope out (record this moment).
5.1.6. Rinsing
- Immerse the endoscope in a basin of clean water and use the pipe cleaning connector and a syringe to rinse each channel as recommended by the manufacturer.
* Note: Do not let chemicals touch the skin and mucosa [2].
5.1.7. Drying
- Dry outside the endoscope, use compressed air to push all the water out of the channels.
- Coat the channels and wipe the outer part with alcohol.
- Dry with compressed air to hang in the cabinet, do not use too strong air pressure; the internal channels of the endoscope may be damaged [1, 2].
5.1.8. Storage
- Endoscope cabinet: Disassemble the valves, hang the endoscopes vertically.
- Cabinetfor preservation are placed in a cool, dry place or specialized cabinets with ventilation fan for preserving the endoscope or the auto-closing cabinets with air-holes on the top and bottom surface, to avoid damaging [1,2]. After each 72 hours, the endoscope needs re-disinfection before reusing.
The disinfectant solution needs to be changed depending on the physicochemical stability of the instruments and the number of procedures carried out. Any cloudy solution should be renewed [1].
5.2. Automated washer and disinfectors
These machines are being used increasingly in endoscopy units and need regular maintenance and control. They have the advantage of proving the standardized disinfection and rinse, heat to optimize the process, filtered tap water, automatic report of washing parameters and a closed system diminishing exposure of fums. However, some crucial cleaning steps need to be performed manually. In addition, the cleaning itself, the check for leaks, the alcohol rinses and forced air drying [1, 2]. The disinfectedprocess by machines is compliedwiththe manufacturer's recommendations.
.jpg)
5.3. Accessories
Reprocessing of material devices is subject of discussion. The complexity of the materials (crevices, wire coils, and retractable components) and the fact that they breach mucosa classify them as critical. These devices need to be sterilized or disposable. The standardized protocol for reprocessing must be strictly followed. Although the cost is higher, using disposable devices should be obtained when possible [1].
Some accessories are difficult to clean can be ultrasonically (> 30kHz) cleaned prior to disinfection or autoclaving. Sterilization should be achieved with water vapor at 134°C for a 134° in 18 minutes, the so-called "prion cycle" [1].
6. ENVIRONMENT
Owing to common adverse reaction to glutaraldehyde, specific criteria relating to exposure have been established. They are defined in terms of average exposure standard and maximum exposure level, which are issued in most countries. The maximum exposure level 0.05 ppm of glutaraldehyde in the UK and 0.2 ppm in France [1].
7. STAFF
Endoscopy staff should be trained not only in the correct use the equipment, but also in case of spillage. All staffs coming into contact with glutaraldehydeneeds to perform yearly lung function test. Gloves and disposable aprons should be worn and changed regularly because they absorb the substance. Staffs is also strongly advised to be vaccinated against hepatitis B [1].
REFERENCES
1. Victor L.F (2008), “Gastrointestinal Endoscopy”, Pediatric gastrointestinal desase, 2(1), 1259-1348.
2. Society of Gastroenterology Nurses and Associates (2012), “Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes”, Inc, 1-20.
3. Nguyễn Thị Bình (2008), “Phương pháp tiệt trùng và bảo quản máy nội soi ống mềm”, Nội soi tiêu hóa, Nhà xuất bản Y học, (3), 21-27.
-

Self-design suction tool
20-05-2021 -

Removing phytobenzoar in Pig's stomach
20-05-2021 -

Remove twisting of the pig colon
04-05-2021 -
Pig stomach endoscopy
04-05-2021
-

Management of Ingested Foreign Bodies in Children: A Clinical Report of the NASPGHAN Endoscopy Committee
28-04-2021 -

Management of Familial Adenomatous Polyposis in Children and Adolescents: Position Paper From the ESPGHAN Polyposis Working Group
28-04-2021 -

Pediatric Colonoscopic Polypectomy Technique
28-04-2021 -

Gastrostomy Placement in Children: Percutaneous Endoscopic Gastrostomy or Laparoscopic Gastrostomy?
28-04-2021







